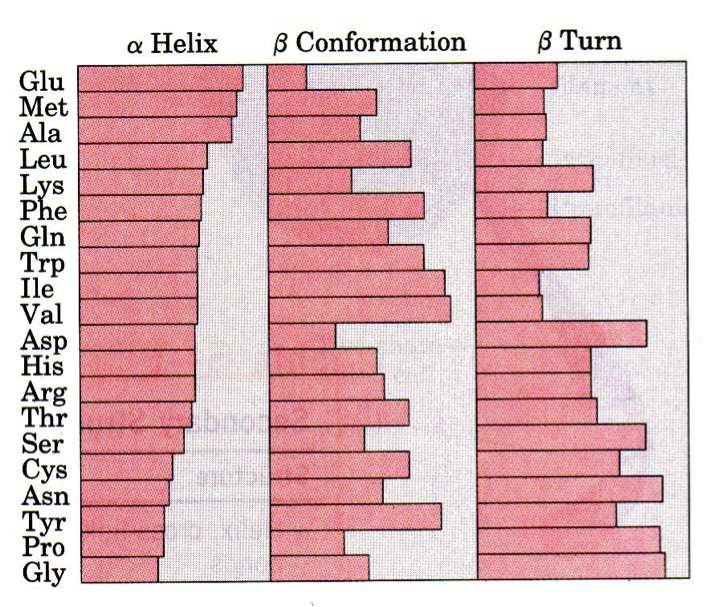
Propensity of AAs to Form Secondary Structures
As we have learned, the order of the AAs is the primary structure and all residues in a polypeptide chain have the same main-chain atoms. What vary are the side chains (R groups). Do the specific AAs present dictate the secondary structure? As shown in the figure, all amino acids can be found in all secondary structure elements, but some are more or less common in certain elements. Pro and Gly, for isntance, aren't good in helices but are favored in beta-turns. If we take this a step further and ask whether 2, 3, or 4 amino acids combinations dictate secondary structure we find a stronger correlation, but still not strong enough to reliably predict tertiary structure.