Secondary Structure (2˚) -- Beta Turns and Random Coils
The Beta Turn
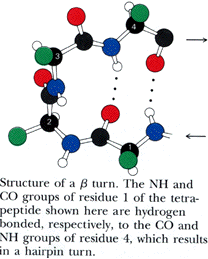 Turns generally occur when the protein chain needs to change direction in order to connect two other elements of secondary structure. The most common is the beta turn, in which the change of direction is executed in the space of four residues. Some commonly observed features of beta turns are a hydrogen bond between the C=O of residue i and the N-H of residue i+3 (i.e, between the first and the fourth residue of the turn) and a strong tendency to involve glycine and/or proline. You will sometimes hear the phrase "beta hairpin" which can be used to describe a beta turn joining two anti-parallel beta strands together. Beta turns are subdivided into numerous types on the basis of the details of their geometry.
Turns generally occur when the protein chain needs to change direction in order to connect two other elements of secondary structure. The most common is the beta turn, in which the change of direction is executed in the space of four residues. Some commonly observed features of beta turns are a hydrogen bond between the C=O of residue i and the N-H of residue i+3 (i.e, between the first and the fourth residue of the turn) and a strong tendency to involve glycine and/or proline. You will sometimes hear the phrase "beta hairpin" which can be used to describe a beta turn joining two anti-parallel beta strands together. Beta turns are subdivided into numerous types on the basis of the details of their geometry.
Gamma turns are three-residue turns which often incorporate a hydrogen bond between the C=O of residue i and the N-H of residue i+2.
Random Coil
Some regions of the protein chain do not form regular secondary structure and are not characterized by any regular hydrogen bonding pattern. These regions are known as random coils and are found in two locations in proteins:
- Terminal arms - both at the N-terminus and the C-terminus of the protein;
- Loops - Loops are unstructured regions found between regular secondary structure elements.
Random coils can be 4 to 20 residues long, although most loops are not longer than 12 residues. Most loops are exposed to the solvent and are have polar or charged side-chains. In some cases loops have a functional role, but in many cases they do not. As a result, loop regions are often poorly conserved (i.e. more prone to change) during evolution.
Some text adapted from: "EXERCISE 3. PROTEIN SECONDARY STRUCTURES" by Kim M. Gernert and Kim M. Kitzler.
