Secondary Structure (2˚) -- Beta Strands
A beta strand is an element of secondary structure in which the protein chain is nearly linear. Adjacent beta strands can hydrogen bond to form a beta sheet (also referred to as a beta pleated sheet). The participating beta strands are not continuous in the primary sequence, and do not even have to be close to each other in the sequence, i.e. the strands forming a beta sheet can be separated in primary structure by long sequences of amino acids that are not part of the sheet. Approximately a quarter of all residues in a typical protein are in beta strands, though this varies greatly between proteins
To view a beta sheet in the KiNG Java Applet, click here. Kinemage 1 shows the 6-stranded parallel beta sheet from domain 1 of lactate dehydrogenase (file 1LDM). This doubly-wound parallel beta sheet is the most common folding pattern found in known protein structures. This "fold" is also known as the "nucleotide-binding domain", because most examples bind a mononucleotide (such as FMN) or a dinucleotide (such as NAD) near the middle of one end of the beta sheet. Lactate dehydrogenase is the classic, first-seen example of this type of structure and has the most frequently-observed topology of beta connections.
Notice that the H-bonds in this parallel shet are slanted in alternate directions, rather than perpendicular to the strands as we will see in antiparallel sheets. Drag right or left to better see that the sheet as a whole twists. This twist is usually described by the twist in orientation of the peptide planes (or H-bond plane) as one progresses along the strand; by this definition beta sheet twist is always right-handed, although by varying amounts. Click on atoms along a strand to tell its direction from the residue numbers, and satisfy yourself that all six strands are indeed parallel. The strand labels show strand sequence order. Note that most sequential pairs are next to each other, and that the chain starts in the middle, moves to one edge, skips back to the middle and then moves out to the other edge.There are three possible ways to form a beta sheet from beta strands, discussed below.
Types of Beta Sheets Observed in Proteins
1) Parallel beta sheet - All bonded strands have the same N to C direction. As a result they have to be separated by long sequence stretches. The hydrogen bonds are equally distanced.
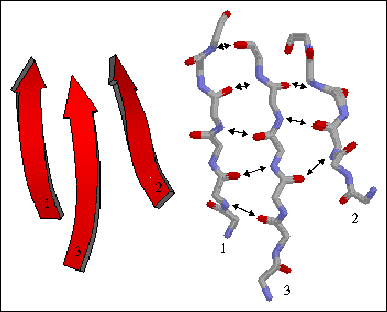
The figure to the left shows a three-stranded parallel beta sheet from the protein thioredoxin. The three parallel strands are shown in both cartoon format (left) and in stick form containing backbone atoms N, CA, C, and O' (right). Hydrogen bonds are identified by arrows connecting the donor nitrogen and acceptor oxygens. Strands are numbered according to their relative position in the polypeptide sequence.
2) Antiparallel beta sheet - The beta strands run in alternating directions and therefore can be quite close on the primary sequence. The distance between successive hydrogen bonds alternates between shorter and longer.
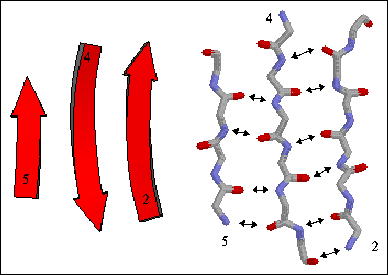
The figure to the right shows a three-stranded antiparallel beta sheet from thioredoxin. The three antiparallel strands are shown in both cartoon format (left) and in stick form containing backbone atoms N, CA, C, and O' (right). Hydrogen bonds are identified by arrows connecting the donor nitrogen and acceptor oxygens. Strands are numbered according to their relative position in the polypeptide sequence.
3) Mixed beta sheet - a mixture of parallel and antiparallel hydrogen bonding. About 20% of all beta sheets are mixed.
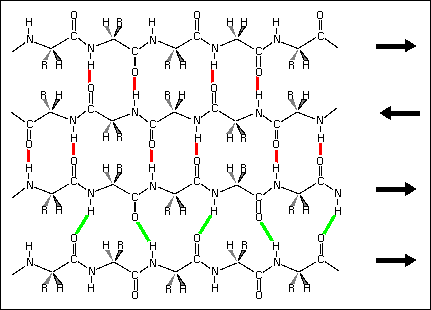
Hydrogen bond patterns in a mixed beta sheet (figure to the left). Here a four-stranded beta sheet containing three antiparallel strands and one parallel strand is drawn schematically. Hydrogen bonds between antiparallel strands are indicated with red lines, those between parallel strands with green lines.
Some of the main features of beta sheets include:
- The extended conformation in a beta strand is about 3.5 Â per residue, and beta strands can be extended as much as 35 Â in length.
- The overall geometry of a sheet is not planar but rather is pleated, with alternating Cα carbons above and below the average plane of the sheet.
- Due to the chirality of the amino acids (L amino acids) all beta strands have a right-handed twist, whereas a beta sheet has an overall left-handed twist.
- Since the strands do not have to be adjacent on the sequence there are many possible ways to arrange strands in a sheet, these arrangements are called topologies and can be quite complicated.
Turn on the side chains in KiNG to examine their arrangment. Along a given strand the sidechains alternate between one side of the sheet (gold) and the other (sea or sky). On adjacent strands the alternation is in register, so that the side chains form rows that are in quite close contact. On parallel beta sheet, the geometry is such that sidechains with branched beta-carbons (Val, Ile, or Thr) make quite favorable contact along a row; since these positions are usually buried and hydrophobic, the result is that Val and Ile are the dominant residues found in these positions. The edge strands, or the very ends of a given strand, can be exposed to solvent and often have significantly more hydrophilic residues (as, for instance, in row 0 here, or the Ser on strand 3).
Some text adapted from: "The Protein Tourist: DOUBLY-WOUND PARALLEL ALPHA/BETA PROTEINS, OR NUCLEOTIDE-BINDING DOMAINS" by J.S. Richardson and D.C. Richardson.
