Secondary Structure (2˚) -- Alpha Helices
While primary structure describes the sequence of amino acids forming a peptide chain, secondary structure refers to the local arrangement of the chain in space. Several common secondary structures have been identified in proteins. These will be described in the following sections and visualized using the KiNG software mentioned previously.
To load the KiNG Java Applet, just click here. Upon loading this page, the KiNG Java Applet should automatically spawn. If you need information on using King, please hover here .
.
The Alpha Helix
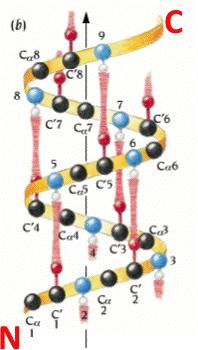 An alpha helix is an element of secondary structure in which the amino acid chain is arranged in a spiral. The kinemage linked above shows an individual alpha helix, viewed from the N-terminal end to resemble the "helical wheel" (see figure below). The O and N atoms of the helix main chain are shown as red and blue balls, respectively. The non-integral, 3.6-residue-per-turn repeat of the alpha helix means that the Cα's of successive turns are about halfway offset, giving the main chain a distinctive 7-pointed star appearance in end view. Notice that the Cα-Cβ bonds do not point out radially from the helix axis but "pinwheel" along the line of one of the adjacent peptides, giving the side chains an asymmetrical start.
An alpha helix is an element of secondary structure in which the amino acid chain is arranged in a spiral. The kinemage linked above shows an individual alpha helix, viewed from the N-terminal end to resemble the "helical wheel" (see figure below). The O and N atoms of the helix main chain are shown as red and blue balls, respectively. The non-integral, 3.6-residue-per-turn repeat of the alpha helix means that the Cα's of successive turns are about halfway offset, giving the main chain a distinctive 7-pointed star appearance in end view. Notice that the Cα-Cβ bonds do not point out radially from the helix axis but "pinwheel" along the line of one of the adjacent peptides, giving the side chains an asymmetrical start.
The hydrophobic side chains are shown in seagreen, polar ones in skyblue, and charged ones in red. These can be turned on by clicking on the checkbox labeled "side ch". Now TURN ON and OFF the various display groups and sets, by clicking in the appropriate button box.
When you clicked the different sidechain types on, what did you observe? Did you notice that the helix has one side with mainly polar residues, and the other with mainly hydrophobic residues?. This is a typical globular-protein helix; in its native configuration, the polar residues would face the solvent while the hydrophobic residues would face the protein interior. In the view menu in KiNG, choose View2 or View3 to see more of the structure.
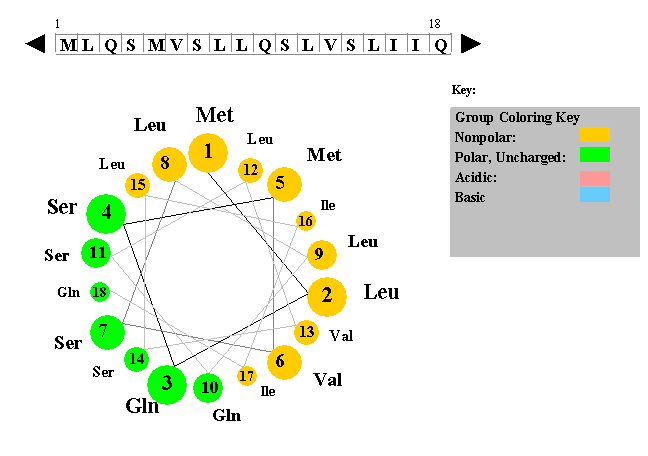 The figure to the left shows a helical wheel representation of an amino acid sequence, as if looking down the axis of an alpha helix that is perpendicular to the page. The amino acid residues are numbered from nearest to most distant and are arranged as an ideal alpha helix with 3.6 residues per complete turn. This figure is a snaphot of a Java Applet written by Edward K. O'Neil and Charles M. Grisham (University of Virginia in Charlottesville, Virginia).
The figure to the left shows a helical wheel representation of an amino acid sequence, as if looking down the axis of an alpha helix that is perpendicular to the page. The amino acid residues are numbered from nearest to most distant and are arranged as an ideal alpha helix with 3.6 residues per complete turn. This figure is a snaphot of a Java Applet written by Edward K. O'Neil and Charles M. Grisham (University of Virginia in Charlottesville, Virginia).
In KiNG, choose View4 for a close-up from the side, with the helical hydrogen bonds (H-bonds) in brown. Turn on "Hbonds" on the button panel, to see the H-bonds in brown. Click on backbone atoms at either end of one of the H-bonds, to verify that the alpha-helical H-bond pattern does indeed go from a donor NH at residue i to an acceptor O at residue i-4 (as shown in the figure to the right). Check to see if this alpha helix has 3.6 residues per turn. If you were to mesure, the rise of a full turn is 5.4 Angstroms (Â).
Alpha helices are nearly all right-handed. To see that this one is righthanded, hold your right hand with the thumb pointing up and the fingers loosely curled; trying to match the spiral of the helix, move slowly along the direction your thumb points and curl along the line of your fingers, as though tightening a screw. When that motion matches the backbone spiral if done with the right hand, then the helix is righthanded.
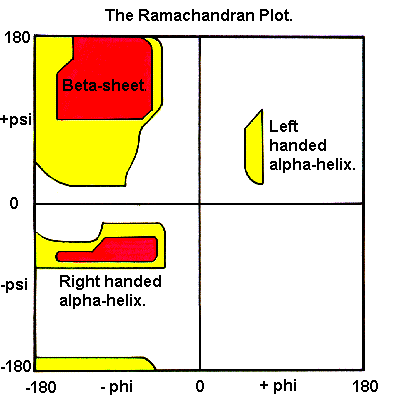 To measure phi,psi angles for the KiNG example helix, turn on "Measure angle & dihedral" on the "Tools" pulldown menu. Start by clicking on a carbonyl C atom near the top, then the next N, then the Cα, and then a C again; at that point the information line will show a dihedral angle that is the phi angle of the central N-Cα bond of those 4 atoms. For a righthanded alpha-helix, it should be in the range of -50 to -80 degrees. Click on the next N and you will get the psi angle, which should be between -25 and -60 degrees. Continue down the helix backbone, getting omega (near 180 degrees), phi, psi, etc. These helical phi,psi values are in the well-populated area in the lower left of the Ramachandran plot (shown on the right).
To measure phi,psi angles for the KiNG example helix, turn on "Measure angle & dihedral" on the "Tools" pulldown menu. Start by clicking on a carbonyl C atom near the top, then the next N, then the Cα, and then a C again; at that point the information line will show a dihedral angle that is the phi angle of the central N-Cα bond of those 4 atoms. For a righthanded alpha-helix, it should be in the range of -50 to -80 degrees. Click on the next N and you will get the psi angle, which should be between -25 and -60 degrees. Continue down the helix backbone, getting omega (near 180 degrees), phi, psi, etc. These helical phi,psi values are in the well-populated area in the lower left of the Ramachandran plot (shown on the right).
In summary, the ideal alpha helix has the following properties:
- It completes one turn every 3.6 residues;
- It rises approximately 5.4 Â with each turn;
- It is a right-handed helix;
- It is held together by hydrogen bonds between the C=O of residue i and the NH of residue i+4;
- It is typically slightly curved.
Some general properties of alpha-helices:
- An average alpha-helix is 10 residues long (15 Â in length), although alpha-helices can range between 4 to 40 residues in length in a standard globular protein.
- All residues participating in an alpha-helix have similar (phi,psi) angles. These angles, which are approximately -60 and -50, are from the bottom left quadrant of the Ramachandran plot.
- Some amino acids are preferred in an alpha-helix. Residues such as Ala, Glu, Leu and Met have a high tendency to participate in a helix , while residues such as Pro and Gly have a small such tendency. Of special interest is Proline, which cannot fit into a helix, and introduces a kink.
- The helix has an overall dipole moment, which is a vector sum of the aligned dipole moments of the individual peptide bonds. The positive pole is at the N-terminus and the negative pole is at the C-terminus. Sometimes this dipole has a functional role.
Some text adapted from: Kinemage Supplement to Branden & Tooze "Introduction to Protein Structure", Chapter 2 - MOTIFS OF PROTEIN STRUCTURE by Jane S. and David C. Richardson.
