Electrophoresis
Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a uniform electric field. Thus it separates components of a mixture based on their size amd/or charge.
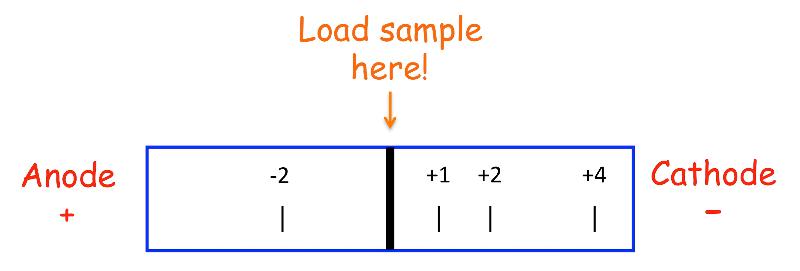
How do you remember which electrode is the cathode and which is the anode?
Simple ... anions travel to the anode and cations travel to the cathode.
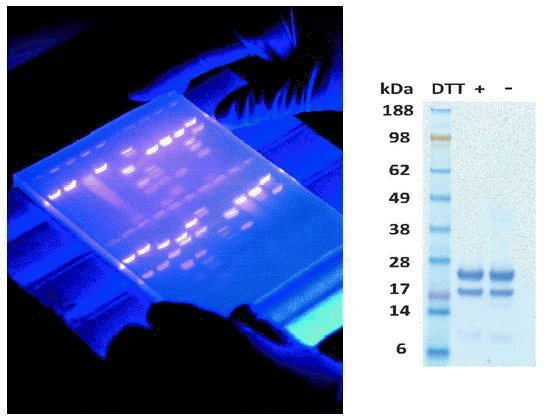
Visualization of proteins on paper or in a gel is an important step in any electrophoresis. Often with DNA the gels are soaked in ethidium bromide which intercalates into DNA and fluoresces under UV light (left image). Proteins may be visualized using silver stain or Coomassie Brilliant Blue dye (right image). In some cases the gels are transferred to a solid support (nitrocellulose) and then probed with specific antibodies (Western Blot).
Paper electrophoresis
Generally used to separate AAs or peptides of differing charge. As shown in the figure, AAs and peptides will separate based on their charge with the most highly charged species moving the furthest.
PAGE (PolyAcrylamide Gel Electrophoresis) -- Native Gel
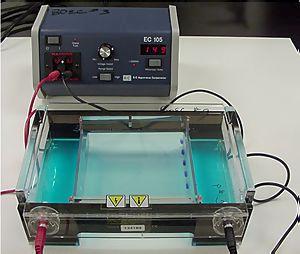 It is used in clinical chemistry to separate proteins by charge and/or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge. It is a process which enables the sorting of molecules. Using an electric field, molecules (such as DNA) can be made to move through a gel made of agar or polyacrylamide. The electric field consists of a negative charge at one end which pushes the molecules through the gel, and a positive charge at the other end that pulls the molecules through the gel. The molecules being sorted are dispensed into a well in the gel material. The gel is placed in an electrophoresis chamber, which is then connected to a power source (see figure to the left). When the electric current is applied, the larger molecules move more slowly through the gel while the smaller molecules move faster. The different sized molecules form distinct bands on the gel.
It is used in clinical chemistry to separate proteins by charge and/or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge. It is a process which enables the sorting of molecules. Using an electric field, molecules (such as DNA) can be made to move through a gel made of agar or polyacrylamide. The electric field consists of a negative charge at one end which pushes the molecules through the gel, and a positive charge at the other end that pulls the molecules through the gel. The molecules being sorted are dispensed into a well in the gel material. The gel is placed in an electrophoresis chamber, which is then connected to a power source (see figure to the left). When the electric current is applied, the larger molecules move more slowly through the gel while the smaller molecules move faster. The different sized molecules form distinct bands on the gel.
The term "gel" in this instance refers to the matrix used to contain, then separate the target molecules. In most cases, the gel is a crosslinked polymer whose composition and porosity is chosen based on the specific weight and composition of the target to be analyzed. When separating proteins or small nucleic acids (DNA, RNA, or oligonucleotides) the gel is usually composed of different concentrations of acrylamide and a cross-linker, producing different sized mesh networks of polyacrylamide. When separating larger nucleic acids (greater than a few hundred bases), the preferred matrix is purified agarose. In both cases, the gel forms a solid, yet porous matrix. Agarose is composed of long unbranched chains of uncharged carbohydrate without cross links resulting in a gel with large pores allowing for the separation of macromolecules and macromolecular complexes.
"Electrophoresis" refers to the electromotive force (EMF) that is used to move the molecules through the gel matrix. By placing the molecules in wells in the gel and applying an electric field, the molecules will move through the matrix at different rates, determined largely by their mass but also their charge and shape which varies widely for proteins. Electrophoretic mobility of small molecules is greater than the mobility of large molecules with the same charge density thus allowing separation. To separate proteins or DNA generally the pH of the buffer and protein mixture is high (~9) so that the proteins carry a net-negative charge. However, because size, charge and shape all play a role in how a molecule will behave in a native gel most scientists use a SDS-PAGE gel which is predictable.
SDS PAGE
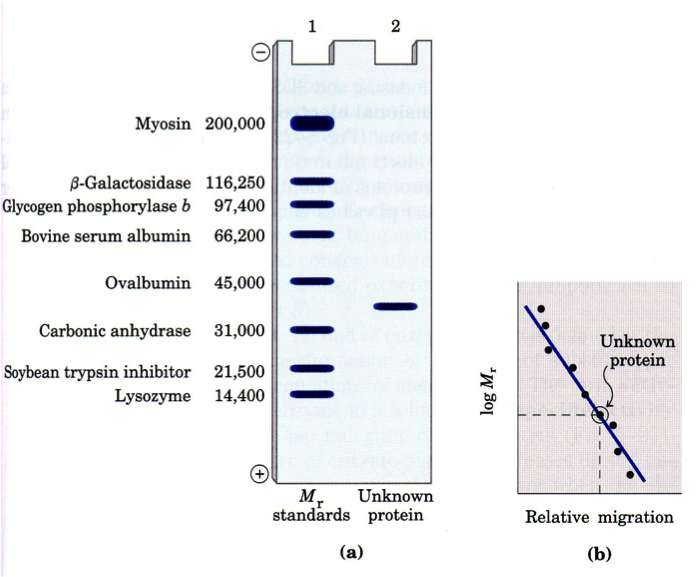 SDS PAGE separate molecules by size because the presence of SDS (sodium dodecyl sulfate) denatures the protein removing 2˚, 3˚ and 4˚ structures (they assume a linear chain) and the SDS coats the molecules giving them a uniform charge/mass ratio. Most often protein samples are also treated with ß-mercaptoethanol (ß-ME) to break any existing disulfide bonds and to give them a linear chain (1˚ structure).
SDS PAGE separate molecules by size because the presence of SDS (sodium dodecyl sulfate) denatures the protein removing 2˚, 3˚ and 4˚ structures (they assume a linear chain) and the SDS coats the molecules giving them a uniform charge/mass ratio. Most often protein samples are also treated with ß-mercaptoethanol (ß-ME) to break any existing disulfide bonds and to give them a linear chain (1˚ structure).
The presence of standards of known size always a calibration curve to be created that can be used to identify the approximate MW of an unknown protein (band).
IEF (IsoElectric Focusing) electrophoresis
Isoelectric focusing (IEF) is a technique for separating different molecules by differences in their isoelectric point (pI). It is a type of electrophoresis, usually performed on proteins in a gel, that takes advantage of the fact that overall charge on the molecule of interest is a function of the pH of its surroundings. When an IEF gel is poured a pH gradient is established
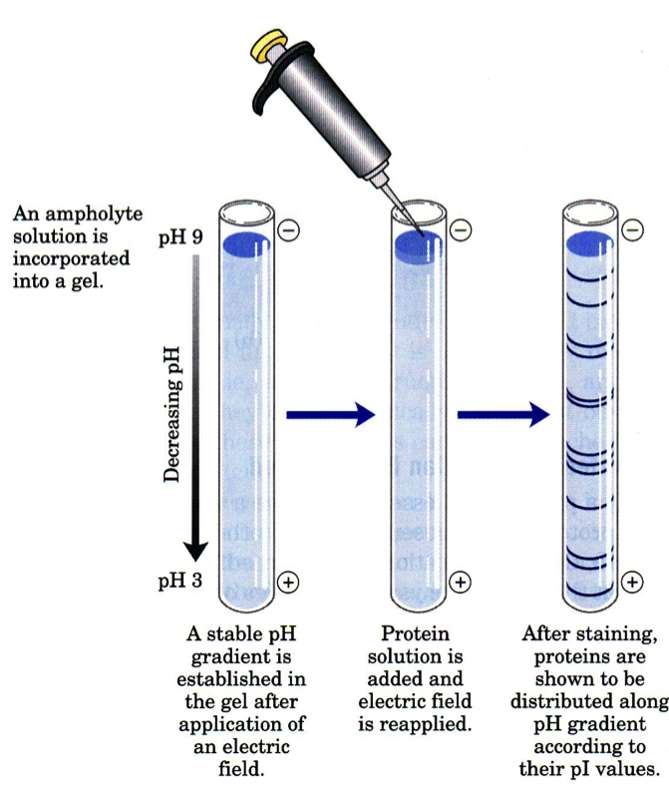
A protein that is in a pH region above its isoelectric point (pI) will be negatively charged and will migrate towards the anode (positive). As it migrates through a gradient of decreasing pH, however, the protein's overall charge will increase until the protein reaches the pH region that corresponds to its pI. At this point it has no net charge and so migration ceases (as there is no electrical attraction towards either electrode). As a result, the proteins become focused into sharp stationary bands with each protein positioned at a point in the pH gradient corresponding to its pI. The technique is capable of extremely high resolution with proteins differing by a single charge being fractionated into separate bands.
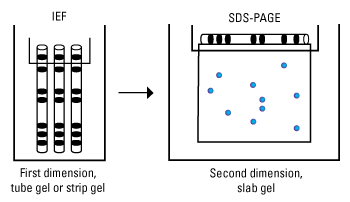
2D Gel Electrophoresis
Two-dimensional gel electrophoresis (2D electrophoresis) is a form of gel electrophoresis commonly used to analyze proteins in which mixtures of proteins are separated by two properties in two dimensions on gels. As shown in the figure, 2D electrophoresis begins with an IEF gel (in a tube) which separates proteins based on their pI. This is then laid on top of a SDS-PAGE gel (90 degrees from the first separation). Because it is unlikely that two molecules will be similar in two distinct properties, molecules are more effectively separated in 2D electrophoresis than in 1D electrophoresis.