Column Chromatography
Column chromatography is one of the most powerful fractionation methods. It can separate components of mixtures based upon:
size (gel filtration/size exclusion)
charge (ion exchange)
affinity (a specific binding affinity)
Commonalities between all three types of chromatography methods is that they all use a resin (solid phase) with special chemical properties held in a glass cylinder (called a "column"). A buffered solution (mobile phase) percolates through the column and is collected in tubes ("fractions") upon exiting the column. A protein mixture is applied in the mobile phase & percolates through through the column as an expanding band. Different proteins migrate differently depending on their properties and those of the resin.
Below we will examine the different forms and examine their particular properties.
Gel Filtration/Size Exclusion
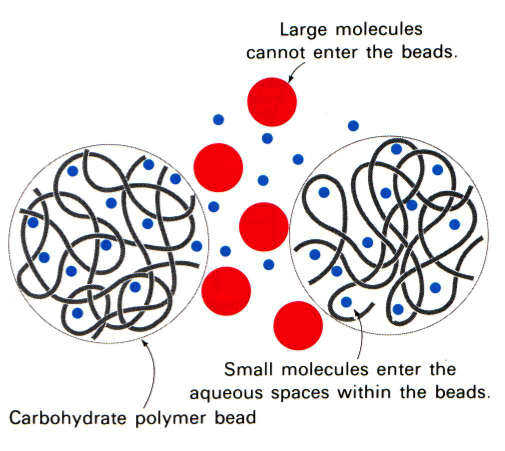 In gel filtration, or as it is sometimes referred to as size exclusion, chromatography the resin are porous (see figure to the left). Some molecules (blue here) can enter the resin and as the lines try to indicate it is not a straight path through; thus it takes longer for small molecules to traverse the column than large molecules which travel around the outside of the resin. This is highlighted in the figure to the right where big molecules (blue) come off first and smaller molecules (red) later.
In gel filtration, or as it is sometimes referred to as size exclusion, chromatography the resin are porous (see figure to the left). Some molecules (blue here) can enter the resin and as the lines try to indicate it is not a straight path through; thus it takes longer for small molecules to traverse the column than large molecules which travel around the outside of the resin. This is highlighted in the figure to the right where big molecules (blue) come off first and smaller molecules (red) later.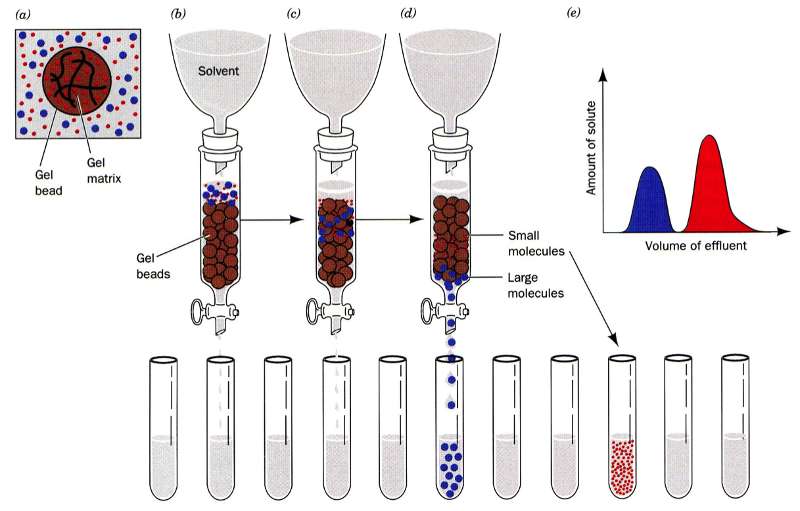
Ion Exchange
Ion exchange chromatography is broken in to two types - anion & cation exchangers. There are many different types of moieties that are used from weakly to very strongly charged thus allowing a huge range of molecules the ability to interact.
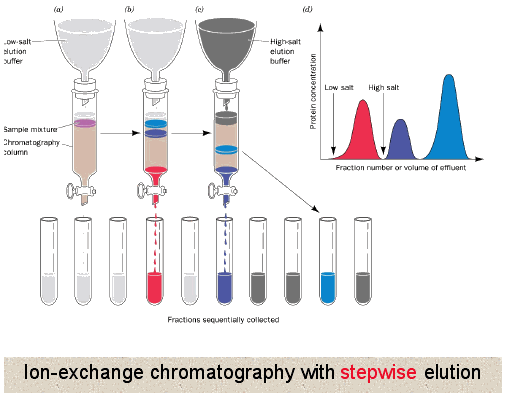
Unlike gel filtration chromatography, here proteins directly interact with the resin. So generally the column is equilibrated in a buffer solution to establish a constant pH in the column, then the protein mixture is loaded where all or some of the proteins interact with the resin depending upon their own charge. Buffer is continued to be applied until all proteins not interacting with the resin have been washed off. At that point usually a gradient of increasing salt concentration (disrupts ionic and hydrogen binding) in the buffer is applied to column allowing the most weakly interacting proteins to release first followed by the more strongly and finally the most strongly interacting. This can also be accomplished by changing the pH of the buffer being applied to the column.
Anion Exchanger
Anion exchanger means that it removes anions from protein mixture so that means the resin must be decorated with positively charged moieties. Before elution begins all positively and uncharged proteins will fall through the column. When you start eluting, first you will knock off the weakly negative proteins (e.g. -1 charge), followed by those with a stronger negative charge (-2), and finally the most negatively charged proteins (-3).
Cation Exchange
It is exactly oppose with a cation exchanger -- here cations are removed from the protein solution so the resin must be negatively charged. Again before elution begins all negatively and uncharged proteins will fall through the column. When you start eluting, first you will knock off the weakly positive proteins (e.g. +1 charge), followed by those with a stronger positive charge (+2), and finally the most positively charged proteins (+3).
Affinity
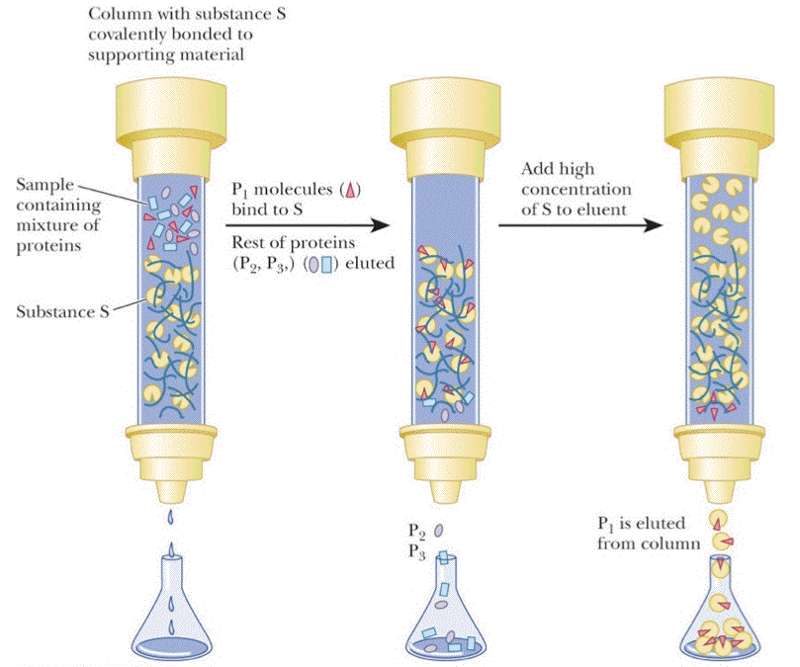
Affinity chromatography requires that you know something specific about your protein -- that it has a specific tag engineered into the sequence, that it binds NAD+, you know the ligand it binds or that you have a specific monoclonal antibody that interacts with your protein. For this type of chromatography your resin is decorated with the antibody, NAD+ or a divalent metal (most popular engineered tag is the 6xHis tag -- 6 His residues at either the N- or C-termini of the protein). Again the column resin is pre-equilibrated in the the appropriate buffer before the protein sample is loaded. It is expected that only the protein of interest will interact. Once all non-interacting proteins have been removed from the column then your protein can be eluted by changing the pH, adding salt, adding a metal chelator, or a high concentration of the ligand.