Domains
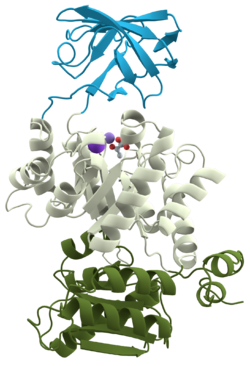 Some of the first proteins utilized in the biochemical laboratory were proteases -- proteins that cleave other proteins. It was frequently observed, however, that a protein would be digested to one or more fragments that could not be digested further except by introducing high temperature or denaturing agents. In 1981 these were named domains and they are now defined as " within a single subunit [polypeptide chain], a contiguous portion which folds into a compact, local semi-independent unit ...". As mentioned, domains are separable by proteases and have frequently been found to have a specific function (binding, digesting, etc). A domain can evolve, function, and maintain its structure relatively independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded, thus they can easily be expressed in a laboratory setting. Typically we find globular proteins to be organized into one or more domains. The ribbon structure (shown to the left) is for pyruvate kinase, a protein with three domains (each colored differently).
Some of the first proteins utilized in the biochemical laboratory were proteases -- proteins that cleave other proteins. It was frequently observed, however, that a protein would be digested to one or more fragments that could not be digested further except by introducing high temperature or denaturing agents. In 1981 these were named domains and they are now defined as " within a single subunit [polypeptide chain], a contiguous portion which folds into a compact, local semi-independent unit ...". As mentioned, domains are separable by proteases and have frequently been found to have a specific function (binding, digesting, etc). A domain can evolve, function, and maintain its structure relatively independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded, thus they can easily be expressed in a laboratory setting. Typically we find globular proteins to be organized into one or more domains. The ribbon structure (shown to the left) is for pyruvate kinase, a protein with three domains (each colored differently).
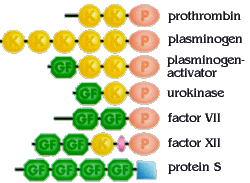
During evolution, DNA segments coding for individual domains of proteins have been duplicated and rearranged. This has given rise to families of related proteins that share similar domains. Blood coagulation factors represent such a family, with members containing similar domains in various combinations and numbers. P=protease domain, GF=growth factor domain, K="kringle"-domain. Coagulation proteins are a great example of proteins being modular.